VALENCE-SHELL ELECTRON-PAIR REPULSION (VSEPR) THEORY AND MOLECULAR SHAPE
Today I was just wandering around and noticed how many biochemical processes are taking place in my surrounding. Each biochemical has its own property, whether physical or chemical each have different molecules and apparently different geometrical shape. The shape of molecules that are interacting with each other is highly specific.
Each and every flavor I taste, the odor or smell I feel, each pharmaceutical product, soaps, detergents, and anything I can touch or feel depends on the part (functional group). Either a molecule wholly that are linked with one another becoming physically and chemically fitted with each other.
The molecular shape is also playing an important role in the functioning of each of the organisms present in the ecosystem. The interacting molecules have complex systems that regulate their nature and different behavioral characteristics such as defensive, navigating, etc. The discussion of various models of structures can be understood by predicting molecular shape with the help of VSEPR theory.
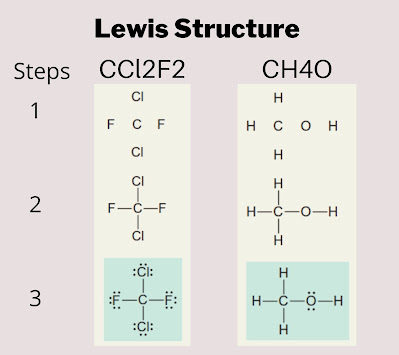
The Lewis structure of molecules is the simplest structure that explains the structure of molecules with the help of valence electrons in an atom. It shows the structural connectivity (bonding of valence electrons) and non-bonding (lone pair) attachments of valence electrons. The following image shows examples of the Lewis structure of the CCl2F2 and CH4O molecules.
Valence-shell electron-pair repulsion (VSEPR) theory is used to formulate the molecular shape from the Lewis structure. This theory is based on the arrangement of each group of valence electrons around a central atom in order to minimize the repulsion and placement of valence electrons as far as possible from each other.
The number of electrons that occupy a confined region surrounding an atom is referred to as a "group" of electrons. A single bond, a double bond, a triple bond, a lone pair, or even a single electron can make up an electronic group.
To enhance the angles between them, each group of valence electrons around an atom repels the other groups. The molecular form is determined by the three-dimensional arrangement of nuclei bound by these groups.
The valence-electron groups, both bonding and nonbonding, around the center atom define the electron-group configuration. The relative positions of the atomic nuclei, on the other hand, define the molecule form. When some of the groups are nonbonding, new molecular structures emerge.
As a result, the identical electron-group arrangement can result in a variety of molecular shapes. Some have only bonding groups, while others with both bonding and non-bonding groups.
We assign each molecule from an AXmEn number to classify it. Here A refers to the central atom, and m and n are integer numbers. X is the surrounding atom and E is the lone pair/ nonbonding electron pair. The angle that is formed between two surrounding atomic nuclei with the nucleus of the A is known as the Bond angle. The ideal bond angles for different compounds' geometry are shown in the image given below
Types of electron group repulsions and molecular shapes:
- The Molecular Shape with Two-Electron Groups
(Linear Molecules) - Molecular Shapes with Three Electron Groups
(Trigonal Planar Molecules) - Molecular Shapes with Four Electron Groups
(Tetrahedral Molecules) - Molecular Shapes with Five Electron Groups (Trigonal Bipyramidal Molecules)
- Molecular Shapes with Six Electron Groups (Octahedral Molecules)
Use of VSEPR Theory to Determine Shape of Molecules:
So, now as we completely understand the theory of molecular shape through VSEPR's model. Let's use a stepwise procedure to obtain a molecular shape from a molecular formula:
step 1: To see the relative positioning of atoms or the number of electron groups, write the Lewis structure from the molecular formula.
Step 2: Assign an electron-group arrangement by counting all bonding and nonbonding electron groups around the core atom.
Step 3: Using the electron-group configuration, predict the ideal bond angle and the direction of any deviation. This deviation is produced by lone pairs or double bonds.
Step 4: Separately count bonding groups and nonbonding groups to draw and label the molecular configuration.







Social Plugin