Classification of Organic Compounds
Chemicals are any chemical substances created by laboratory or industrial chemical operations. Pure substances or combinations of substances can be used. Organic and inorganic chemicals are the two types of chemicals.
Inorganic chemistry deals with the other elements of the periodic table and their compounds, whereas organic chemistry deals with almost all carbon-containing substances.
Organic chemistry covers roughly 19 million known carbon compounds, much outnumbering inorganic molecules (about 500,000). The ability of carbon to create branched chains and ring structures with other carbon atoms is responsible for this vast outnumbering.
The phrase "organic chemistry" originated in 1806 by Swedish chemist J. Berzelius. It was limited to substances that could be made by living creatures in the early nineteenth century. F. Wohler, on the other hand, challenged the idea that ‘organic substances could not be synthesized in 1828. Wohler was able to synthesize urea from inorganic sources.
Scientists didn't recognize that carbon content is the defining characteristic of an organic compound until the mid-19th century. The differences between these two fields – organic and inorganic chemistry – are currently becoming increasingly confused. But, the mechanistic studies and structure of their materials both differ distinctively.
The International Union of Pure and Applied Chemistry (IUPAC) standards govern the naming of organic compounds, which are outlined in its "Blue Book."
There are different classifications of organic compounds based on carbon chains and attached functional groups. From simple hydrocarbons to complex macromolecules, each organic compound has its own structural and chemical properties.
The compounds consisting of carbon chain skeletons are classified into
• Acyclic/open-chain compounds
• Cyclic/ closed chain compounds
The flowchart below shows the classification of organic compounds.
• Acyclic/open-chain compounds
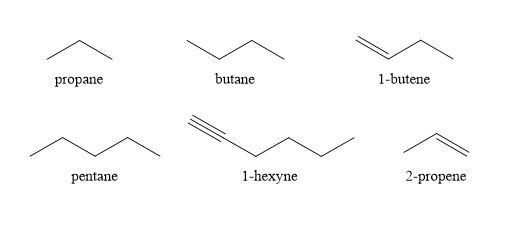
The open-chain compounds consist of saturated (alkane) and unsaturated compounds (alkenes and alkynes). These open chained compounds can be single or branched chains.
Examples:
Single chained compounds
Propane (C3H8): CH3- CH2- CH3
Butane (C4H10): CH3- CH2- CH2- CH3
Branched-chain compounds
• Cyclic/ closed chain compounds
Cyclic or closed chain compounds refer to ring structures that can be homocyclic or heterocyclic compounds. This division is based on the type of atoms that a compound contains.
If the compound ring contains Only carbon atoms it is a Homocyclic compound. Or if the cyclic structure contains any heteroatom such as nitrogen, oxygen, sulfur, etc it is a heterocyclic compound.
1. Homocyclic compounds
These carbon compounds are ring structures, that can be of two types:
• Alicyclic compounds
Alicyclic compounds are related to Aliphatic compounds in terms of their property resemblance. One or more all-carbon rings that can be saturated or unsaturated are referred to as alicyclic compounds. Examples of such compounds are cyclobutene, cyclohexene, etc.
• Aromatic compounds
As the name suggests Aromatic is derived from ‘Aroma’ a Greek word that means smell or flavor. These compounds have a delocalized Pi electron system that is not present in aliphatic compounds.
It can be single-ringed, or one or more benzene rings can fuse together to form such compounds.
There are further two types of aromatic compounds,
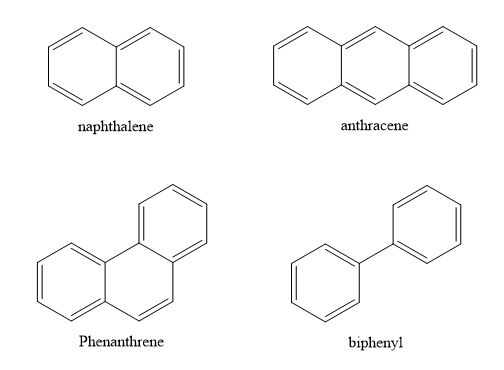
- Benzenoid Aromatic compounds
These are the aromatic compounds that only contain the benzene ring and its derivatives. They can be monocyclic, bicyclic, tricyclic, etc, depending on the number of rings they contain.
Monocyclic aromatics
Polycyclic aromatics (bi- tri-)
- Non-Benzenoid Aromatic compound
The aromatic compounds that do not have any benzene ring, instead they contain highly unsaturated rings are called non-Benzenoid aromatics.
For example:
2. Heterocyclic compounds
The compounds which contain different heteroatom along with carbon in the ring structure are Heterocyclic compounds. They can also be alicyclic or aromatic based on the delocalization of pi electrons. Examples of such compounds are given below,
- Applications of Organic compounds:
The uses of Organic compounds are increasing day by day as they serve us in a variety of ways. There are many applications of these compounds and these are becoming essential for our life.
There are many products that we are using in our daily routine, made of organic compounds such as,
- Drugs and pharmaceutical products
- Perfumes
- Lotions (daily skincare to medicated creams or lotions)
- Organic foods and additives
- The synthetic fabric (mainly polyester, nylon, etc)
- Paints and enamels
- Soaps and shampoos
- Lipsticks/ nail polish remover /makeup products
- Coal
- Natural gas
- Fertilizers/ insect repellants
- Enzymes
- Vitamins
- Dyes (hair colors/ fabric dyes)
- Mothballs
- Cleaners
- Batteries
- Baking soda/ washing soda
- Polymers (PVC, polyethylene, nylon, cellulose, etc)
- Plastics
- Medicine
- Surfactants
- Petrol
- Biofuel
Soap and detergent are two separate types of organic chemicals, despite the fact that they are both used for washing. The saponification reaction produces glycerol and crude soap when hydroxide combines with a compound (such as animal fat).
While soap is an emulsifier, detergents are surfactants that lower the surface tension of water. It also improves the solubility of organic substances, making them ideal for oily, greasy surfaces.
The process of fractional distillation is used to separate organic compounds from the raw material by the difference in their boiling points. This technique is also used at a large scale for the production of petrochemicals from petroleum or crude oil.
The make-up industry is nothing without cosmetic products. There are many low-quality cosmetics that damage the skin, for this cure the chemist plays an important role.
The changes in the skin are examined carefully and the aspects of the environment on the skin are considered to make such products that are effective for skin problems. Such products are used to beautify and enhance skin care.
• If you want to study more applications enlisted with compound names and structure visit the List of 100 organic compounds and their applications.











Social Plugin