As we know, spectroscopy is the interaction of EM waves with the matter, Infrared spectroscopy is the study of the infrared region of the electromagnetic spectrum.
It has a longer wavelength and lower frequency than visible light. It includes a variety of techniques, the majority of which are based on absorption spectroscopy. It can be used to identify and study chemicals, just like any other spectroscopic technique. A Fourier transform infrared (FTIR) spectrometer is a common laboratory instrument that employs this technique.
There are three regions that lie in the Infrared part of electromagnetic spectra;
Near IR: This region has higher energy than the other regions which are approx 14000-4000 cm-1 (0.8-2.5 μm wavelength). It is used to study the overtones and harmonic vibrations
Mid IR: It lies in between the near and far IR Region it ranges approximately 4000-400 cm-1 ( 2.5-25 μm ). It can be used to study the fundamental vibrations that are associated with the rotational vibrations of atoms.
Far IR: This region is associated with the least energy region that has approximately 400-10 cm-1 energy and 25-300 μm wavelength. This region lies adjacent to the microwave region of spectra. It is used to study the rotational spectra of heavy atoms.
Introduction
Infrared radiation was first discovered by William Herschel in the year 1800. He was investigating the energy level linked to the wavelength in the noticeable range. He noticed that the temperature increased from the glowing blue to the red region of the spectrum. He also assessed the temperature outdoors in the red region, thinking that the increase should stop. However, the temperature there was clearly even higher. He called these rays infrared rays of invisible rays.
The very first infrared spectrometer was built in 1835.
Absorption of infrared is fixed to compounds with small energy variations in the possible vibrational and rotational states. For a chemical to absorb infrared, the vibrations and rotations within a molecule must cause a change in the dipole of the molecular compound. The bonds between atoms vibrate.
A photon of light that has a frequency in the infrared range will be absorbed if the bonds between atoms in the target material allow these atoms to vibrate at these frequencies. In other words, infrared light will be absorbed by materials that allow for the oscillation at these frequencies.
CONDITIONS FOR IR SPECTROSCOPY
In IR activity requires changes in the permanent dipole for a vibrational mode in a molecule.
IR radiation must have the same frequency as the vibration of a specific group or bond. Even if it has no dipole moment, it will produce some vibrations that induce a dipole.
In order to find out whether a molecule is IR active or not, you must look at the following factors.
Types Of vibrational modes in Infrared spectroscopy
Vibrational modes are the type of vibrations that occur in a molecule. There are two main types of vibrations that are discussed in the following topic. One is fundamental vibrations and the other is molecular vibrations.
Molecular Vibrations:
The vibrations in a molecule as a result of the absorption of IR radiation that results in atomic vibration. There are two types of molecular vibrations:
Stretching vibrationsIn these vibrations, the atoms move along the bond axis, and because of this movement, the bond length increases or decreases at regular intervals which gives rise to a change in dipole moment. There are two types of stretching vibrations,
- Symmetrical stretching
When two bond lengths of a molecule increase or decrease simultaneously this type of stretching is known as symmetrical stretching.
- Asymmetrical stretching
It is the opposite of symmetrical stretching in this type of stretching one bond length increases or the other decreases.
Bending vibrationsBending vibrations does not involve the bond length variation but it results in deformation and alters the bond angle of the molecule. There are two types of bending vibrations; In-plane and out-of-plane bending.
- In-plane bending
Scissoring
Rocking
- Out-plane bending
Wagging
Twisting
Fundamental Vibrations:
A linear molecule has 3N - 5 vibrational modes, while a nonlinear molecule has 3N - 6 vibrational modes. These modes are also known as degrees of freedom.
Stretching vibrations; The number of stretching vibrations in both linear and nonlinear molecules is equal to (n-1) while in Bending vibrations;The number of bending vibrations in a nonlinear molecule is (2n-5) and in a linear molecule, it is equal to (2n-4).
As an example, A non-linear triatomic molecule H2O molecule has 3 vibrational frequencies.
Whereas, There are 3 atoms in the carbon dioxide but 4 modes of vibration as the CO2 is a linear molecule (3n-5).
Simple diatomic molecules have a single bond and only one vibrational band. When a molecule is symmetrical, such as Nitrogen (N2), the band appears only in the Raman spectrum, not in the IR spectrum. Asymmetric diatomic molecules, such as Carbon monoxide (CO), absorb in the infrared region. Because more complex molecules have more bonds, their vibrational spectra are also more complicated. Such spectra contain more peaks than usual.
Types of IR Spectroscopy
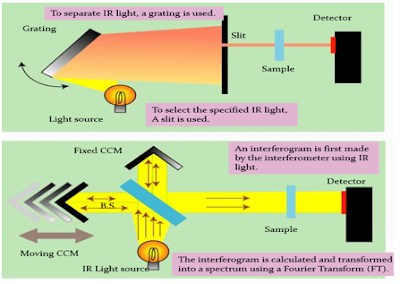
An infrared spectrophotometer is a device that uses infrared light to pass through an organic molecule and generates a spectrum that shows the amount of light transmitted from the molecule.
The main components of an IR spectrometer include an IR source, diffraction gratings, mirrors, a sample chamber (consisting of reference and the analyzing sample), and a detector. The IR light source can be a deuterium lamp, tungsten filament lamp, or xenon arc lamp.
FTIR is Fourier Transform Infrared Spectroscopy is a technique of measurement to collect IR spectra of molecules. The main components that are involved in the instrumentation are a light source, Interferometer, Interferogram, Detector, and mathematical Fourier transform.
The main difference between both types of IR spectroscopy is that the dispersive method includes a monochromator. Also, it is slow and expensive. However FTIR uses an interferometer and it is cheap, convenient, and faster.
Fingerprint IR Region
It is extremely difficult to assign all the absorption bands in 1450 to 600 cm-1 region of the IR spectrum; thus, it is known as the fingerprint region due to the unique pattern found there.
The number of bending vibrations in this region is greater than stretching vibrations. Slight differences in the structure and constituents of a molecule can alter the absorption bands of molecules in this region. The absorption bands of many compounds in the region are useful in identifying the compounds.
Advantages and Applications of FTIR
It is a non-destructive method that is faster, within a few seconds the frequencies can be measured. This technique works with more sensitivity and enables low noise and fast scanning detectors. It has very simple instrumentation and there is very less chance of mechanical breakdown.
It ensures quality and quantity controls and is a very reliable technique to identify a sample.
This technique is used for,
Determination of purity:
The purity of a compound is determined by comparing the spectrums. The peaks in the spectrum can identify if any impurity is present in the compound.
Identification of Organic compounds:
Organic compounds can be identified by the presence of peaks at specific functional group regions. For example presence of the OH, the group will result in a broad peak at 3700 cm-1, etc.
Determining the structure of molecule:
The determination of the symmetry and shape of molecules can be done with the help of the FTIR spectrum. For example; the IR spectrum of NO2 has three peaks at 750, 1323, & 1515 cm−1
Study of chemical reaction:
The type of chemical reaction can be easily understood by the appearance and disappearance of peaks. For example, if a compound used as the reactant contains an OH group, but after the completion of the reaction the hydroxyl group is removed, the peak of the OH group will disappear.
• Other applications
It can also be used to identify the presence of Water in sample molecules, measure the composition of paints and varnishes, the age of artifacts can also be known by using this technique. The bond variations in the structure of molecules that make specific tautomers are also identified by FTIR.
Is there any Limitation to Infrared Spectroscopy?
The molecular weight of a substance cannot be determined using IR spectroscopy. IR spectroscopy, in general, does not reveal the relative positions of different functional groups on a molecule. It is impossible to tell whether an unknown substance is a pure compound or a mixture of compounds based on a single IR spectrum. A mixture of paraffin and alcohols, for example, will produce the same IR spectra as higher molecular weight alcohols.






Social Plugin